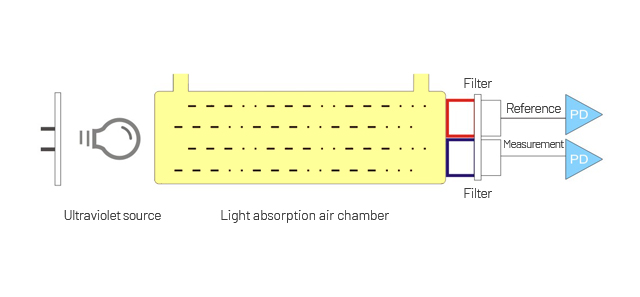
The characteristics of light in the ultra-violet region of the spectrum lead to molecular electronic transitions when the light is absorbed. Absorption of ultraviolet photons excites the electrons of the atoms within the molecule to a higher energy state. The excited electrons quickly loose the energy by returning to the ground state by one of four methods; dissociation, where absorption of high-energy photons can cause the electron to leave the molecule completely, causing it to fragment; re-emission, where an identical photon is re-emitted as the electron decays back to its ground state; fluorescence, where a photon is emitted at a lower frequency than the original absorption as the electron decays back to its ground state, causing the gas to appear to glow.
 EMAIL
EMAIL
